Brain Structure in Bilingual Compared to Monolingual Individuals with Alzheimer’s Disease: Proof of Concept
Journal of Alzheimer’s Disease 76 (2020) 275–280 DOI 10.3233/JAD-200200 IOS Press
Cyrus A. Raji,a,1,∗, Somayeh Meysamib,1, David A. Merrillc,d, Verna R. Porterb,d and Mario F. Mendezb,c,e, aMallinckrodt Institute of Radiology, Division of Neuroradiology, Washington University in St. Louis, St. Louis, MO, USA bDepartment of Neurology, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA, cDepartment of Psychiatry & Biobehavioral Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA, dThe John Wayne Cancer Institute and Pacific Neuroscience Institute, Providence and St. Johns Health Center, Santa Monica, CA, USA, eV.A. Greater Los Angeles Healthcare System, Los Angeles, CA, USA.
ABSTRACT
Background: Bilingualism is increasingly recognized as protective in persons at risk for Alzheimer’s disease (AD).
Objective: Compare MRI measured brain volumes in matched bilinguals versus monolinguals with AD.
Methods: This IRB approved study analyzed T1 volumetric brain MRIs of patients with criteria-supported Probable AD.We identified 17 sequential bilinguals (any native language) with Probable AD, matched to 28 (62%) monolinguals on age and MMSE. Brain volumes were quantified with Neuroreader®. Regional volumes as fraction of total intracranial volume (TIV) were compared between both groups, and Cohen’s D effect sizes were calculated for statistically significant structures. Partial correlations between bilingualism and brain volumes adjusted for age, gender, and TIV.
Results: Bilinguals had higher brain volumes in 37 structures. Statistical significance (p < 0.05) was observed in brainstem (t = 2.33, p = 0.02, Cohen’s D= 0.71) and ventral diencephalon (t = 3.01, p = 0.004, Cohen’s D= 0.91). Partial correlations showed statistical significance between bilingualism and larger volumes in brainstem (rp =0.37, p = 0.01), thalamus (rp = 0.31, p = 0.04), ventral diencephalon (rp = 0.50, p = 0.001), and pallidum (rp = 0.38, p = 0.01). Bilingualism positively correlated with hippocampal volume, though not statistically significant (rp = 0.17, p = 0.26). No brain volumes were larger in monolinguals. Conclusion: Bilinguals demonstrated larger thalamic, ventral diencephalon, and brainstem volumes compared to matched monolinguals with AD. This may represent a neural substrate for increased cognitive reserve in bilingualism. Future studies should extrapolate this finding into cognitively normal persons at risk for AD.
Keywords: Alzheimer’s disease, bilingual, brain structure, Neuroreader®, volumetrics, brain atrophy.
1These co-authors contributed equally to this work.
∗Correspondence to: Cyrus A. Raji, MD, PhD, Mallinckrodt Institute of Radiology, Division of Neuroradiology, Washington University in St. Louis, St. Louis, MO, USA. E-mail: cyrusraji@gmail.com.
INTRODUCTION
Given continued unfortunate failures to develop successful disease-modifying treatments for AD, there is increased motivation to find modifiable environmental risk factors or preventative measures [1].
Bilingualism recognized as a way of delaying clinical AD
Investigators have increasingly recognized bilingualism, or the ability to use more than one language on a daily basis, as one of the most important modifiable risk factors for delaying the expression of clinical AD [2]. If symptoms of dementia could be postponed through promoting bilingualism, even for a few years, there could be major savings in both human costs and health economic considerations [3]. In fact, some, but not all studies, report a four-to-five year delay in onset of the clinical symptoms of dementia despite comparable neuropathology [4, 5]. The underlying neurobiological mechanisms for this influence of bilingualism on the expression of dementia remain unclear.
Cognitive reserve may be a potential explanation
Cognitive reserve involves the optimization of cognitive strategies and brain resources through education, occupational attainment, and engagement in social or stimulating activities [6]. Bilingualism increases cognitive reserve through the constant management of two simultaneously active and competing languages [7, 8]. Pervasive multilingualism could be a factor in the low prevalence estimates of cognitive complaints and dementia in older adults in Luxembourg [9], and, in the Lothian Birth Cohort (Scotland), those who learned a second language achieved better cognitive results independent of childhood intelligence [10]. Others report that the larger the number of languages spoken, the better the cognitive performance in the oldest old [11]. Brain structure is recognized as a key metric of cognitive reserve with larger brain volumes denoting increased reserve and lower risk for dementia [12].
The purpose of this study was to therefore compare brain volumes, a marker of cognitive reserve, in bilingual and monolingual persons with AD who were matched on age of onset/presentation and overall severity. Understanding this question will help determine the underlying influence of bilingualism on cognitive reserve and its relevance for AD risk reduction.
Given that about half of the world’s population has some degree of bilingualism [13], incorporating bilingualism as a prevention strategy for AD can have widespread implications.
METHODS
All participants in this study had Probable AD as determined by National Institute on Aging- Alzheimer’s Association (NIA-AA) Criteria for “Probable AD”.
Subject demographics in bilingualism versus monolingualism
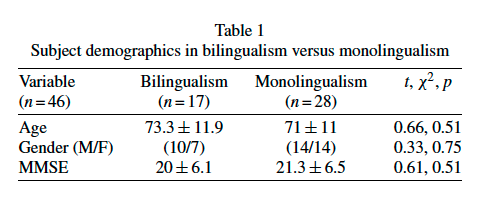
In many cases, the NIA-AA criteria were applied retrospectively to patients diagnosed by older criteria. We identified 17 patients with bilingualism and Probable AD. All had sequential bilingualism (English learned after age 5) with various native languages. In addition, all of these patients were immigrants who used English primarily outside the home and were reported by caregivers or family as having the premorbid ability to converse in both languages. These 17 patients were matched for age of presentation within six years and global severity of cognitive impairment with the Mini-Mental State Examination (MMSE) [14]. Subject demographics of the bilingual and monolingual groups are detailed in Table 1.
MRI methods
Data from research participants, particularly the brain MRI scans, were analyzed as part of an IRB approved protocol (IRB# 16-001491). Each participant underwent brain MRI including a 3D volumetric MPRAGE sequence on a 3.0 Tesla Siemens Scanner.
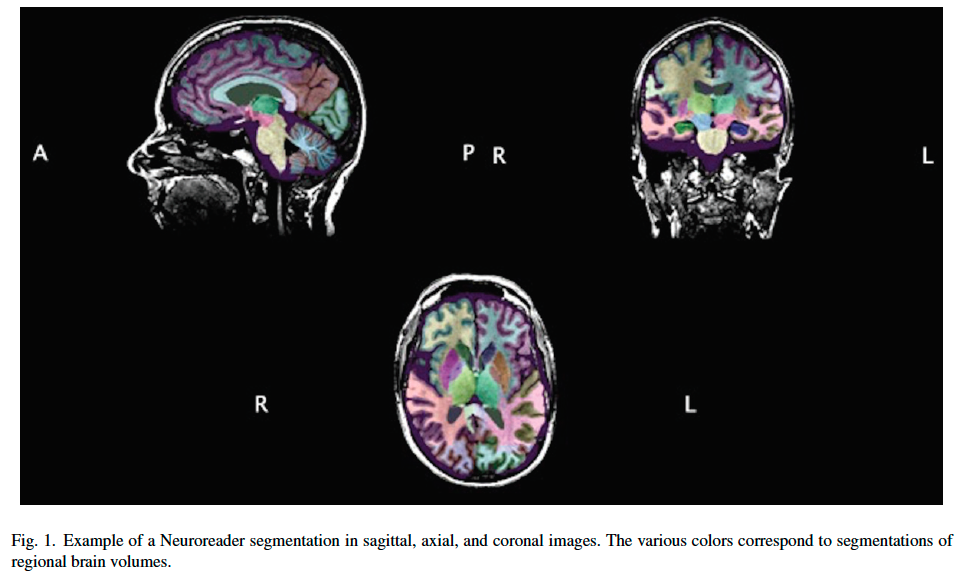
Scans were then analyzed with an FDA cleared volumetric program (Neuroreader®, Aalborg, Denmark), as detailed in prior work [15, 16]. In each scan, 45 brain structures were quantified including the hippocampus, lobar structures, subcortical regions (thalamus, caudate, putamen, etc.), ventral diencephalon, midbrain, ventricular, and white matter volumes with an atlas-based segmentation, also detailed in prior work [16]. Total gray matter, white matter, and cerebrospinal fluid volumes were also computed. Total intracranial volume (TIV) was calculated from the sum of these three volumes and regional brain volumes are also generated as a fraction of TIV. The following metrics were therefore computed for each of the 45 structures: 1) Regional brain volume in ml; 2) the ratio of a region of interest volume to TIV; 3) the number of standard deviations from the normative database (NR index); 4) the number for standard deviations from the mean scaled between –2 and +2 (Z-score); and 5) percentile of comparison to the normative database. The normative database was drawn from control subjects from the Alzheimer’s Disease Neuroimaging Initiative (n = 231) with an age range of 60–90 and 53% women, 47% men. All normative database comparisons for computing Z-scores and percentiles were adjusted for age, gender, and TIV in a multiple regression model. A typical example of a Neuroreader® segmented brain is noted in Fig. 1.
RESULTS
Bilingualism was related to multiple larger brain volumes with statistical significance or trends. Statistically significant t-test results had Cohen’s D effect sizes that were either moderate or large.
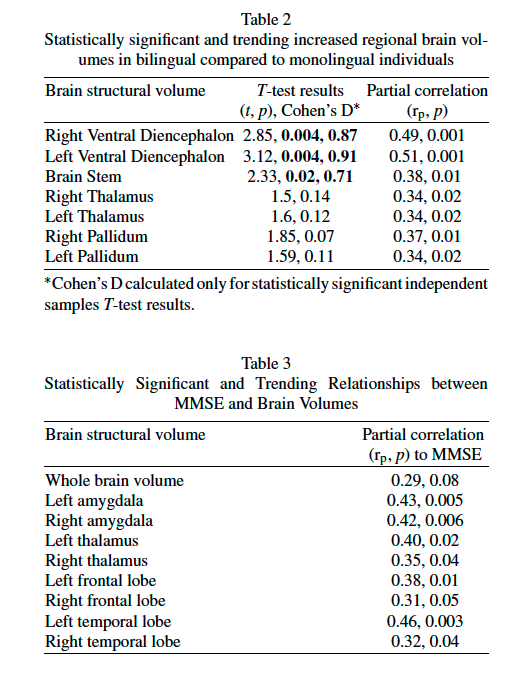
Table 2 details these findings with several brain regions showing statistical significance despite adjusting for age, gender, and TIV co-variates. These areas were the right ventral diencephalon (rp = 0.49, p = 0.001), left ventral diencephalon (rp = 0.51, p = 0.001), brainstem (rp = 0.38, p = 0.01), right thalamus (rp = 0.34, p = 0.02), left thalamus (rp = 0.34, p = 0.02), right pallidum (rp = 0.37, p = 0.01), and left pallidum (rp = 0.34, p = 0.02). Notably, these brain structures are not correlated to MMSE itself. Rather, the structures that were statistically significant or trending in correlation with MMSE are detailed in Table 3. These were whole brain volume (rp = 0.29, p = 0.08), left amygdala (rp = 0.43, p = 0.005), right amygdala (rp = 0.42, p = 0.006), left thalamus (rp = 0.40, p = 0.02), right thalamus (rp = 0.35, p = 0.04), left frontal lobe (rp = 0.38, p = 0.01), right frontal lobe (rp = 0.31, p = 0.05), left temporal lobe (rp = 0.46, p = 0.003), and right temporal lobe (rp = 0.32, p = 0.04).
No statistically significant interactions were noted between bilingualism and MMSE on brain structure. No other brain structures showed statistically significant relationships with bilingualism or MMSE, including the hippocampus. Also, the monolingual group did not show any statistically significant higher regional brain volumes on MRI compared to the bilingual group. Also, there was no correlation between left versus right hippocampal asymmetry or temporal lobe asymmetry.
DISCUSSION
In this study, we have demonstrated that for a comparable level of cognitive impairment, bilinguals with AD demonstrated multiple increased regional brain volumes compared to monolinguals with AD.
These brain regions may function as neural correlates for increased resistance for AD pathology in bilingualism. Additionally, the results suggest that bilingualism has potential as a preventive strategy for AD given its relation to larger brain volume correlates of cognitive reserve.
Prior studies of normal individuals have suggested structural and functional changes in bilingualism in language areas and their related connections. Several studies have shown increased gray matter volume in cognitively-normal bilinguals especially left hemisphere frontal regions and their connections for language functions and language control [7, 17, 18].
Bilinguals have more gray and white matter in some areas compared to monolinguals
Compared to normal monolinguals, normal bilinguals have more gray matter in these areas, particularly left inferior frontal gyrus, and greater frontal white matter tracts [19, 20]. Normal bilinguals compared to normal monolinguals have more white matter in the corpus callosum, the inferior and superior longitudinal (L >R) fasciculi, and other white matter tracts, especially with L2 proficiency and immersion [21, 22]. Early-acquired, proficient bilinguals have increased structural and functional connectivity between inferior frontal gyrus and regions of the brain supporting language control such as the salience and frontoparietal networks, between white matter regions mediating connectivity in nonverbal executive control tasks, and between right and left inferior frontal gyri [23, 24]. Long-term structural changes in gray and white matter connectivity, may also occur when the second language is acquired relatively late in life [19, 20].
In contrast to relatively preserved language areas and their related connections, when bilinguals develop dementia or AD, they may have greater mesiotemporal neuropathology or hypometabolism at comparable levels of functional clinical impairment [24, 25]. One of these studies compared monolingual (34 MCI; 13 AD) with multilingual (34 MCI; 13 AD) patients and found higher tissue densities in medial temporal areas in the multilingual MCI group, but similar or lower in the multilingual AD group [24]. However, in language and language control areas, both of the multilingual groups had thicker cortex than the monolingual groups [25, 26]. Our results add to these findings, indicating that key subcortical areas are also better preserved among AD patients who speak more than one language compared to those who only speak one.
The advantages of this study were a characterized bilingual and monolingual AD cohort, matched for age of presentation and general cognitive impairment, with automated volumetrics that assessed regions not typically evaluated such as the ventral diencephalon.
The ventral diencephalon has been previously implicated in neurodegeneration and cognitive reserve [27] and this structure includes the mammillary bodies that are part of hippocampal circuitry and has connections to the nucleus basalis of Meynert of the basal forebrain. The implication of the brainstem as increased in bilingualism is also relevant to AD pathogenesis given its inclusion of the locus coeruleus that also been implicated in early AD neuropathology [28]. Disadvantages and thus topics for future studies include inclusion of functional imaging sequences and longitudinal data on cognitive decline.
The degree of brain atrophy in individuals ‘matched’ for cognitive decline remains controversial and an area of active research. Several studies have found that among ‘matched’ individuals, the group with greater cognitive reserve, bilingual cohorts included, have greater brain atrophy for the same degree of cognitive decline [2, 29]. These differences in the studies may be due to the fact that the protective effect of cognitive reserve rapidly declines with greater degree of brain atrophy resulting in rapid deterioration after a ‘tipping point’ [30]. Additional topics for future directions can be to engage in amyloid and tau PET imaging as well as imaging of synaptic vesicles as different molecular imaging metrics of AD pathology and cognitive reserve. Hippocampal subfield segmentation as well as segmentation of language areas are also other approaches of possible future analyses. Overall, this study lends insight with quantitative brain MRI into novel neural correlates of higher cognitive reserve in bilinguals with AD.
In conclusion, this study adds to the literature on differences in brain structures between bilingual and monolingual patients with AD. In addition to the literature on greater mesiotemporal impairment at comparable levels of dementia, there may be relative preservation of critical subcortical structures, along with language areas and their connections, consistent with improved neural reserve with bilingualism.
Future studies of neuroimaging in these patients are needed to further clarify the neuroimaging structures that mediate neural reserve and the effects of bilingualism in delaying the onset of the clinicalsymptoms of AD.
ACKNOWLEDGMENTS
Supported by McLoughlin Cognitive Health Gift Fund and the Pituitary Injury Foundation. Dr. Raji is supported in his research by grants from the WUSTL NIH KL2 Grant (KL2 TR000450 – ICTS Multidisciplinary Clinical Research Career Development Program), the Radiological Society of North America Research Scholar Grant and the Foundation of the American Society of Neuroradiology Boerger Research Fund for Alzheimer’s Disease and Neurocognitive Disorders. Dr. Mendez is PI on US National Institute on Aging Grant 1RF1AG050967.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0200r2).
REFERENCES
[1] Sommerlad A, LivingstonG(2017) Preventing Alzheimer’sdementia. BMJ 359, j5667.
[2] Mendez MF (2019) Bilingualism and dementia: cognitivereserve to linguistic competency. J Alzheimers Dis 71, 377–388.
[3] Bialystok E, Abutalebi J, Bak TH, Burke DM, Kroll JF (2016) Aging in two languages: Implications for public health. Ageing Res Rev 27, 56–60.
[4] Mukadam N, Sommerlad A, Livingston G (2017) The relationship of bilingualism compared to monolingualism to the risk of cognitive decline or dementia: a systematic review and meta-analysis. J Alzheimers Dis 58, 45–54.
[5] Klimova B, Valis M, Kuca K (2017) Bilingualism as a strategy to delay the onset of Alzheimer’s disease. Clin Interv Aging 12, 1731–1737.
[6] Stern RA, Silva SG, Chaisson N, EvansDL(1996) Influence of cognitive reserve on neuropsychological functioning in asymptomatic human immunodeficiency virus-1 infection. Arch Neurol 53, 148–153.
[7] DeLuca V, Rothman J, Bialystok E, Pliatsikas C (2019) Redefining bilingualism as a spectrum of experiences that differentially affects brain structure and function. Proc Natl Acad Sci U S A 116, 7565–7574.
[8] Lombardi G, Polito C, Berti V, Bagnoli S, Nacmias B, Pupi
A, Sorbi S (2018) Contribution of bilingualism to cognitive reserve of an Italian literature professor at high risk for Alzheimer’s disease. J Alzheimers Dis 66, 1389–1395.
[9] Perquin M, Diederich N, Pastore J, Lair M-L, Stranges S, Vaillant M, on behalf of the MemoVie Group (2015) Prevalence of dementia and cognitive complaints in the context of high cognitive reserve: a population-based study. PLoS One 10, e0138818.
[10] Bak TH, Nissan JJ, Allerhand MM, Deary IJ (2014) Does bilingualism influence cognitive aging? Ann Neurol 75, 959–963.
[11] Kav´e G, Eyal N, Shorek A, Cohen-Mansfield J (2008) Multilingualism and cognitive state in the oldest old. Psychol Aging 23, 70–78.
[12] Fenu G, Lorefice L, Arru M, SechiV, Loi L, Contu F, Cabras F, Coghe G, Frau J, Fronza M, Sbrescia G, Lai V, Boi M, Mallus S, Murru S, Porcu A, Barracciu MA, Marrosu MG, Cocco E (2018) Cognition in multiple sclerosis: Between cognitive reserve and brain volume. J Neurol Sci 386, 19–22.
[13] (2019) Half of the world is bilingual. What’s our problem? The Washington Post.
[14] Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state: A practical method grading the cognitive state of patients for the clinician. Psychiatr Res 12, 189–198.
[15] Raji CA, Merrill DA, Barrio JR, Omalu B, Small GW (2016) Progressive focal gray matter volume loss in a 280 C.A. Raji et al. / Brain Structure in Bilingual Compared to Monolingual Individuals with AD former high school football player: a possible magnetic resonance imaging volumetric signature for chronic traumatic encephalopathy. Am J Geriatr Psychiatry 24, 784–790.
[16] Meysami S, Raji CA, Merrill DA, Porter VR, Mendez MF (2019) MRI volumetric quantification in persons with a history of traumatic brain injury and cognitive impairment. J Alzheimers Dis 72, 293–300.
[17] Bilingual and monolingual brains compared: a functional magnetic resonance imaging investigation of syntactic processing and a possible “neural signature” of bilingualism. J Cogn Neurosci 1, 153-169.
[18] Cognitive control for language switching in bilinguals: A quantitative meta-analysis of functional neuroimaging studies. Lang Cogn Process 27, 1479-1488.
[19] Grundy JG, Anderson JAE, Bialystok E (2017) Neural correlates of cognitive processing in monolinguals and bilinguals: Neural correlates of bilingualism. Ann N Y Acad Sci 1396, 183–201.
[20] Abutalebi J, Guidi L, Borsa V, Canini M, Della Rosa PA, Parris BA,Weekes BS (2015) Bilingualism provides a neural reserve for aging populations. Neuropsychologia 69, 201–210.
[21] Pliatsikas C, Moschopoulou E, Saddy JD (2015) The effects of bilingualism on the white matter structure of the brain. Proc Natl Acad Sci U S A 112, 1334–1337.
[22] Luk G, Bialystok E, Craik FIM, Grady CL (2011) Lifelong bilingualism maintains white matter integrity in older adults. J Neurosci 31, 16808–16813.
[23] Perani D, Farsad M, Ballarini T, Lubian F, Malpetti M, Fracchetti A, Magnani G, March A, Abutalebi J (2017) The impact of bilingualism on brain reserve and metabolic connectivity in Alzheimer’s dementia. Proc Natl Acad Sci U S A 114, 1690–1695.
[24] Berken JA, Chai X, Chen J-K, Gracco VL, Klein D (2016) Effects of Early and late bilingualism on resting-state functional connectivity. J Neurosci 36, 1165–1172.
[25] Duncan HD, Nikelski J, Pilon R, Steffener J, Chertkow H, Phillips NA (2018) Structural brain differences between monolingual and multilingual patients with mild cognitive impairment and Alzheimer disease: Evidence for cognitive reserve. Neuropsychologia 109, 270–282.
[26] Schweizer TA, Ware J, Fischer CE, Craik FIM, Bialystok E (2012) Bilingualism as a contributor to cognitive reserve: Evidence from brain atrophy in Alzheimer’s disease. Cortex 48, 991–996.
[27] Lebedeva AK,Westman E, Borza T, Beyer MK, Engedal K, Aarsland D, Selbaek G, HabergAK(2017) MRI-based classification models in prediction of mild cognitive impairment and dementia in late-life depression. Front Aging Neurosci 9, 13.
[28] Del Cerro I, Villarreal MF, Abulafia C, Duarte-Abritta B, S´anchez SM, Castro MN, Bocaccio H, Ferrer I, Mench´on JM, Sevlever G, Nemeroff CB, Soriano-Mas C, Guinjoan SM (2020) Disrupted functional connectivity of the locus coeruleus in healthy adults with parental history of Alzheimer’s disease. J Psychiatr Res 123, 81–88.
[29] Costumero V, Marin-Marin L, Calabria M, Belloch V, Escudero J, Baquero M, Hernandez M, Ruiz de Miras J, Costa A, ParcetM-A, A´ vila C (2020) A cross-sectional and longitudinal study on the protective effect of bilingualism against dementia using brain atrophy and cognitive measures. Alz Res Therapy 12, 11.
[30] Mungas D, Gavett B, Fletcher E, Farias ST, DeCarli C, Reed B (2018) Education amplifies brain atrophy effect on cognitive decline: implications for cognitive reserve. Neurobiol Aging 68, 142–150.
ISSN 1387-2877/20/© 2020 – IOS Press and the authors. All rights reserved 276 C.A. Raji et al. / Brain Structure in Bilingual Compared to Monolingual Individuals with AD.